Noteworthy – Valproate Scandal: Parents feel failed by State over drug that harmed their children.
THE CHIILDREN – THE IRISH STATE WANTS TO FORGET – THE VALPROATE CHILDREN. OACS Ireland wants to be clear we want the Irish Inquiry into EPILIM (SODIUM VALPROATE) TO COMMENCE WITH URGENCY !
Irish Families have been through enough. It is now time for Ireland to own up to the harm done by a drug EPILIM (SODIUM VALPROATE). Women have lost babies and had miscarriages they also had there whole lives turned upside down due to the consequences of Epilim (Sodium Valproate) families now they deserve answers.

NOTEWORTHY – Valproate scandal: Parents feel failed by State over drug that harmed their children.
Noteworthy finds that pleas for help from women impacted by valproate (Epilim) continue to be dismissed. BY MARIA DELANEY APRIL 16TH, 2021 19 MIN READ. https://bit.ly/3x63Tbl
“MY WORLD FELL apart that day when I saw the news. It was telling me something I knew in my head and my heart, but I hoped that I was wrong.”
Debbie Adams flicked on TV while grabbing her nightly meds at a wedding abroad in 2017. She was devastated by a report from the UK which revealed that the dangers of women using the epilepsy drug sodium valproate – sold under the brand name Epilim in Ireland – had been kept from patients for decades.
At her clinic appointment in an Irish hospital just six months earlier, she asked the neurologist she saw that day – she saw different doctors at the clinic almost every time she attended – if there was any connection between her taking valproate and the developmental problems that her three- and five-year-old daughters were experiencing. [The doctor] turned away from me and said ‘there is no correlation between Epilim and these conditions’.
What was revealed:
- No timeframe for inquiry promised by the Minister for Health.
- On findings of international reports, expert psychiatrist says Ireland “is no different”.
- The recommended ‘full suite of services’ for affected children is non-existent, resulting in families paying thousands for therapies and other costs.
- Nurses required for full implementation of a pregnancy prevention programme and national response to valproate not appointed.
- Valproate pregnancy registry delayed due to the pandemic.
- Promised stakeholder group to examine effectiveness of risk minimisation measures not established.
- Patient groups concerned some women at risk are still not being informed.
HERE –
You can see all the documents “unpublished and published” which has been released under FOI via Maria Delaney we would like to give great thanks to Maria for never giving up on families impacted by this drug.
JOINT HEALTH COMMITTEE REPORT on Foetal Anti-Convulsant Syndrome May 2018.

The Committee wishes to emphasise its support for those affected by FACS and acknowledges their concerns as advocated by the FACS Forum. This report makes a number of recommendations which are intended to address some of these concerns. A lack of appropriate preventative measures resulted in numerous cases of FACS in Ireland. It will take time and resources to examine these areas. However, the Committee are anxious that some areas are addressed as a matter of urgency. The Committee urges that prompt access to adequate services is made available to families affected by FACS. The Committee also recommends that all stakeholders collaborate to ensure these families receive this support.
It is noted that individuals with FACS are not affected by chance but by the failure to adequately inform and counsel women who were prescribed valproate medicines. Further examination is required to establish liability but regardless of the causes, the Committee is of the opinion that the State has responsibility to assist all those affected by FACS. Dr. Michael Harty, T.D. Chair Joint Committee on Health 30 May 2018 https://bit.ly/3mZbfsz
RAPID ASSESSMENT REPORT of the number of women and children exposed to sodium valproate in Ireland 1975-2015. Released – August 2018.
Written by Ronan Glynn is an Irish public health physician who has served as Deputy Chief Medical Officer of Ireland since October 2018 and Head of the Health Protection Unit at the Department of Health. He previously served as Acting Chief Medical Officer of Ireland from July to October 2020
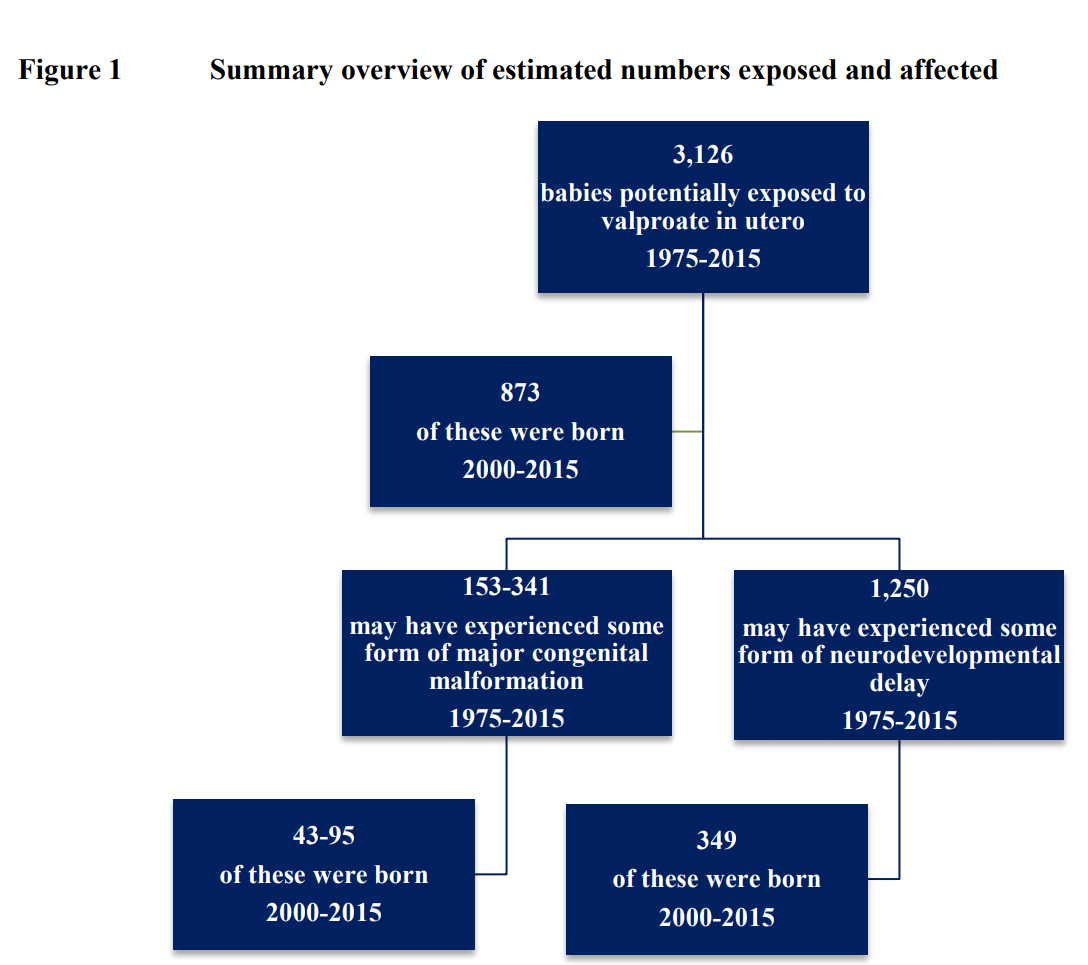
It is estimated that, between 1975 and 2015, inclusive, approximately 3,083 (3,058 epilepsy; 25 other indications) maternities were in women who were taking valproate when becoming pregnant. It is estimated that, between 1975 and 2015, inclusive, approximately 3,126 (3,100 epilepsy; 26 other indications) babies were potentially exposed to valproate in utero.
Of these, it is estimated that 873 were born between 2000 and 2015, inclusive. These will now be aged 2-18 years. A similar number of children, currently aged 0-16, were potentially exposed in the years from 2002-2017, inclusive. On the basis of the above estimations and on emerging international data regarding rates of major congenital malformation and neurodevelopmental delay following exposure to valproate in utero, it is estimated that between 1975 and 2015, between 153 and 341 children will have experienced a major congenital malformation and up to 1,250 children will have experienced some form of neurodevelopmental delay. Of children born since 2000, it is estimated that between 43 and 95 will have experienced a major congenital malformation and 349 will have experienced some form of neurodevelopmental delay; a similar number of children, born between 2002 and 2017 and currently aged 0-16, are likely to have experienced such a malformation and/or delay. https://bit.ly/3ttoAf8
THE “UNPUBLISHED REPORT” BY THE HEALTH SERVICE EXECUTIVE VALPROATE. 10/1/2019.
Response Project. This report provides a high-Level outline of the aim and objectives of the HSE Valproate Response Project as commissioned by the Chief Clinical Officer. Received under FOI on the latest. https://bit.ly/3ts60nP
Project Objectives
- The project created a comprehensive action plan to achieve the following objectives:
- Ensure women of childbearing age who continue/commence taking Epilim (Sodium

- Valproate) are monitored, subject to annual specialist review & have access to specialist services when required.
- Support the implementation of the HPRA guidance on the pregnancy prevention programme.
- Ensure that people who may have been impacted by current or historic risks of Epilim exposure in the womb are provided with information and support.
- Estimate the number of children/adults who may have been impacted by exposure to Epilim in the womb (Foetal Anticonvulsant Syndrome, FACS).
- Develop a diagnostic pathway for children/adults who may have FACS.
- Develop an assessment and intervention (or care) pathway for those children and adults diagnosed with FACS.
- Scope support needs for children/adults diagnosed with FACS, & make recommendations regarding Disability/Mental Health Services.
- To establish a Programme for Women’s Health in Epilepsy.
- Implement media and communication strategy to provide information and support to patients.

THE DEPARTMENT OF HEALTH “Unpublished BRIEFING -2020“. To the Joint Health Committee.
A Department of Health briefing to the Joint Committee in November 2020, obtained by Noteworthy through a Freedom of Information (FOI) request, lists the actions taken in response to the recommendations. For this particular action, it wrote that “the HSE has had ongoing engagement with families affected by FACS/FVS since 2018”. Received under FOI on the latest.
BACKGROUND:
Sodium valproate is an oral medication which has been licensed and prescribed worldwide since the 1970s, primarily for the treatment of epilepsy. There is evidence that some anti-epileptic drugs (AEDs), including sodium valproate, are teratogenic (a drug, or other substance, capable of interfering with the development of a foetus, causing birth defects and developmental difficulties through life), and are associated with an increased risk of foetal valproate syndrome (FVS). FVS is an umbrella term for a range of conditions that can affect some babies exposed to AEDs while in utero. Based on the totality of available scientific data, it is now known that children exposed to sodium valproate in utero are at a high risk of serious developmental disorders (in up to 30-40% of cases) and congenital malformations (in approximately 10% of cases).https://bit.ly/3dqkwqr
Donnelly commits to inquiry into anti-epilepsy drug linked to birth defects. Valproate-containing medicines can cause birth defects and developmental disorders.

Nov 24th 2020, 10:50 AM 18,703 Views Health Minister Stephen Donnelly:
HEALTH MINISTER STEPHEN Donnelly has committed to holding an inquiry into the historical licencing and use of the anti-epileptic drug sodium valproate (Epilim) in Ireland.
This decision follows a meeting with representatives from Epilepsy Ireland the the Organisation for Anti-Convulsant Syndrome Ireland.
Valproate-containing medicines can cause birth defects and developmental disorders in children whose mothers take such medicines during pregnancy. Read more here on how you can support a major Noteworthy investigation into why Irish women were not told for decades about an epilepsy drug that causes birth defects. 24.11.2020 – Donnelly commits to inquiry into anti-epilepsy drug linked to birth defects
Women ‘dismissed’ by doctors over mesh and epilepsy drug fears ‘deserve apology’ 08.07.2020 –
Women ‘dismissed’ by doctors over mesh and epilepsy drug fears ‘deserve apology’
Survey finds one in six women taking epilepsy medication don’t know the risk it poses to pregnancy.
A NEW SURVEY carried out by Epilepsy Ireland has found 17% of women who are taking the epilepsy drug sodium valproate (Epilim) are unaware that the drug can cause serious birth defects. The study also showed that one in three (33%) are unaware that when taken in pregnancy, the drug can cause learning and developmental problems in children. 03.07.2020 – Survey finds one in six women taking epilepsy medication don’t know the risk it poses to pregnancy
Please do not stop taking your medication without your healthcare professional advice.

God I am flabbergasted at the effect of this med I’m on for years