The HPRA #MedSafetyWeek – “Every Report Counts”

The Health Products Regulatory Authority, are seeking support for the fifth annual #MedSafetyWeek, which sees 74 medicine regulatory authorities across the world taking part in a social media campaign to raise awareness of suspected side effects from medicines, and the importance of reporting them. The theme of this year’s campaign is “Every Report Counts”. The HPRA are asking stakeholders and partner agencies to support this message by sharing the key messages and visuals as appropriate throughout the week-long campaign, running from 2 – 8 November. Chairperson of OACS Ireland said “MedSafetyWeek is the upmost importance especially for those who suffered harm. It is the time for raising awareness of harm”.

The campaign forms part of a global initiative led by the Uppsala Monitoring Centre (UMC) – the World Health Organisation (WHO) Collaborating Centre for International Drug Monitoring – in collaboration with the Heads of Medicines Agencies (HMA) and the International Coalition of Medicines Regulatory Authorities (ICMRA). Some of us will not experience any problems when using medicines. All medicines have some risks and side effects can happen.
WHAT IS IMPORTANT – The Irish Regulator, such as the HPRA rely on the reporting of suspected side effects to make sure medicines on the market are as safe as possible and do what they are intended to do.
The HPRA would very much appreciate the support of organisations to help raise awareness of this important public health campaign in Ireland. They understand that this may not be possible in all cases but if we are in a position to lend them our backing, then the HPRA are asking organisations to visit there social media channels over the course of the week to share the campaign content. It is also possible to download generic versions of the animations these can be used on our / your social media channels.
The campaign will consist primarily of short animations on the social media channels of the participating national medicines agencies, including the HPRA’s Twitter, LinkedIn and Instagram accounts.
Check out the HPRA press release, which highlights there participation in #MedSafetyWeek 2020, it will appear on the home page of the HPRA website on Monday morning the 2nd of November 2020.
Sinead Curran, Director of Human Products Monitoring, stated: “Patient safety is always a top priority for the HPRA. We hope that this important campaign encourages everyone to report suspected side effects from medicines. Reporting is invaluable as it allows us to learn more about new or known risks of medicines. Every report counts and contributes to improving the safety of medicines for all patients.”
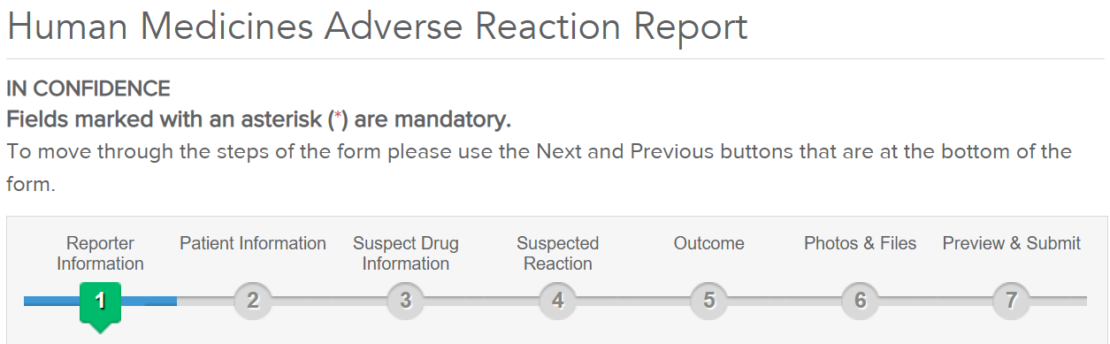
Please see link and picture below for Patients, carers, and including their healthcare professionals are asked to REPORT their suspicions of side effects through our online report form here: https://bit.ly/2HMcr2y
Please remember there is NO SAFE dose of Epilim (Sodium Valproate) in Pregnancy.
Finally, do NOT STOP taking your medication without your Healthcare Professional advice.