Access to Product Information for Anticonvulsant Medicines Authorised in Ireland
*The active substance is the main ingredient contained in every medicine. For example, the active substance in Epilim is sodium valproate. The active substance in every medicine is listed on the outer packaging and in the package leaflet (PL), usually under the brand name.
| Active Substance* | Brands available in Ireland include: | Product Information** available from HPRA or EMA website |
| Carbamazepine | Tegretol – Carbamazepine Essential | HPRA |
| Clobazam | Clobazam – Frisium Thame – Epaclob – Perizam | HPRA |
| Clonazepam | -Clonazepam Rosemont -Rivotril | HPRA |
| Ethosuximide | -Zarontin | HPRA |
| Gabapentin | Gabapentin -Gabapentin Rosemont -Gabin -Neurontin -Neurotil | HPRA |
| Lacosamide | Lacosamide Accord -Lacosamide Krka -Lacosamide UCB -Vimpat | EMA |
| Lamotrigine | -Lamictal -Lamoro -Larig | HPRA |
| Levetiracetam | Keppra -Levetiracetam Accord -Levetiracetam Actavis -Levetiracetam Bluefish -Levetiracetam Brillpharm -Levetiracetam Clonmel -Levetiracetam Ratiopharm -Levetiracetam Sun -Levetiracetam Teva -Levetiracetam Thame -Levetiracetam Wockhardt -Matever | EMA (Keppra, Levetiracetam Accord, Levetiracetam Actavis, Levetiracetam Ratiopharm, Sun, Teva, Matever) HPRA (Levetiracetam Bluefish, Levetiracetam Brillpharm, Levetiracetam Clonmel, Levetiracetam Wockhardt) |
| Oxcarbazepine | Trileptal | HPRA |
| Phenytoin | Epanutin | HPRA |
| Phenobarbital | Phenobarbital | HPRA |
| Pregabalin | Brieka -Lyrica -Mypreg -Pregablin -Pregablin Accord – Pregablin Clonmel – Pregablin Krka – Pregablin Mylan – Pregablin Pfizer – Pregablin Sandoz – Pregablin Sandoz GmbH – Pregablin Teva – Pregablin Wockhardt – Pregablin Zentiva | HPRA Brieka, Mypreg, Pregablin, Pregablin Clonmel, Pregablin Krka, Pregablin Teva, Pregablin Wockhardt EMA Lyrica, Pregablin Accord, Pregablin Mylan, Pregablin Pfizer, Pregablin Sandoz, Pregablin Sandoz GmbH, Pregablin Zentiva |
| Primidone | Mysoline | HPRA |
| Rufinamide | Inovelon | EMA |
| Sodium Valproate | Epilim | HPRA |
| Tiagabine | Gabitril | HPRA |
| Topiramate | Topamax | HPRA |
| Vigabatrin | Kigabeq -Sabril | EMA (Kigabeq) HPRA (Sabril) |
| Zonisamide | Zonegran -Zonisamide Mylan | EMA |
**Product information for a medicine is made up of the Summary of Product Characteristics (SmPC) and Package Leaflet (PL). These documents are issued when a medicine is first licensed for use and are reviewed and updated as necessary throughout the lifetime of a medicine, to reflect the current knowledge about a medicine and risks associated with its use. The Summary Product Information (SmPC) is mainly intended for use by healthcare professionals and the Patient Information Leaflet (PIL) has more comprehensive information presented in an abbreviated and easy to read format for patients.
Educational Materials are one of the measures used to describe safety concerns about a medicines to healthcare professionals and patients. The materials focus on one or more specific safety concern related to use of the medicine. They provide information on specific risks and actions required to minimise/prevent such risks. Examples of educational materials for healthcare professionals include dosing and administration guides, prescriber checklists etc. Examples of such materials for patients include patient alert cards, patient guides and patient reminder cards.
Educational materials for valproate are accessible via the link below. At present, there are no educational materials in place for any of the other currently authorised anticonvulsant medicines. http://www.hpra.ie/homepage/medicines/special-topics/valproate-(epilim)/educational-materials-toolkit
Searching for product information/educational materials on the HPRA website
SmPCs, PLs and educational materials (if available) for medicines currently authorised in Ireland are accessible via the HPRA website. Please follow the following instructions:
- Go to the HPRA website www.hpra.ie
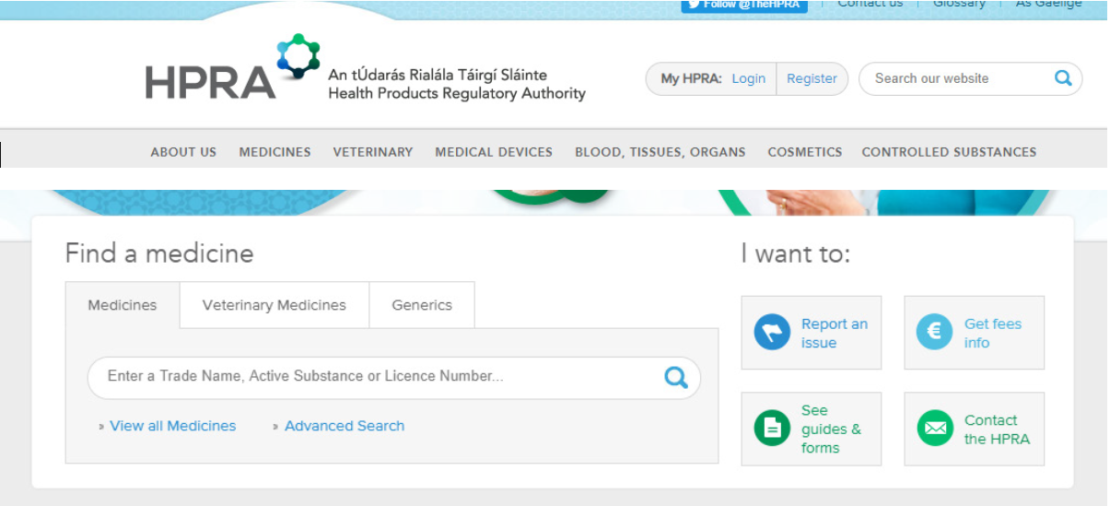
- Use the ‘Find a medicine’ search function on the homepage (See Figure 1 below)
- Type in the medicine name (brand name or active substance) where it says ‘Enter a Trade Name, Active Substance or Licence Number’ and press enter
- All the results will be listed and the product information (and educational materials if relevant) are listed on the right hand side under ‘Documents’. Click on ‘SPC’ or ‘PIL’ and the documents will open as a PDF document.
- Please see figure below.
Figure 1 – Search function on HPRA website www.hpra.ie
Searching for product information on the EMA website
Product information for medicines authorised via the centralised application procedure, co-ordinated by the European Medicines Agency (EMA) is available on the EMA website. If you search for a medicine on the HPRA website as per above, when you click on SPC or PIL under ‘Documents’, you will automatically be transferred to the EMA website for such products.
- Once on the EMA website (www.ema.europa.eu), search for the medicine using the search box available (See figure 2 below)
- Click on the Human medicine European public assessment report (EPAR) for the medicine you wish to access product information for
- Click on product information under the ‘Table of Contents’
- Click on ‘EPAR – Product Information’ to open a PDF document of the SmPC and PIL.
Figure 2 – Search function on EMA website

It is very important that you do NOT STOP taking you medication without speaking to your Health Care Professional.
